Contents:
- Overview
- Procedure
- Benefits and advantages
- Multiple sclerosis treatment
- Rheumatoid arthritis treatment
- Related scientific publications
T-lymphocytes play a pivotal role in adaptive immunity. Pathogenic T cell activity is instrumental to the development of acquired immunological disorders. Self-reactive T lymphocytes able to attack host tissues induce autoimmune inflammation. High activity of allergen-specific T lymphocytes underlies the pathogenesis of allergic diseases. In addition, various abnormalities in regulatory T cell activity often contribute to disease development. Therefore, the pathogenetic treatment of immune disorders should be based on targeted modulation of T-lymphocyte activity.
Variable regions of T-cell receptors (TCRs) form in the postnatal period, such that no innate immune tolerance exists to TCRs which makes TCR immunogenic for the host immune system. Specifically, the immunogenic TCR regions are called idiotypic epitopes (Figure 1), which are recognized by a idiotype-specific (anti-idiotypiс) variable region of another TCR in the context of the major histocompatibility complex molecules (MHC).
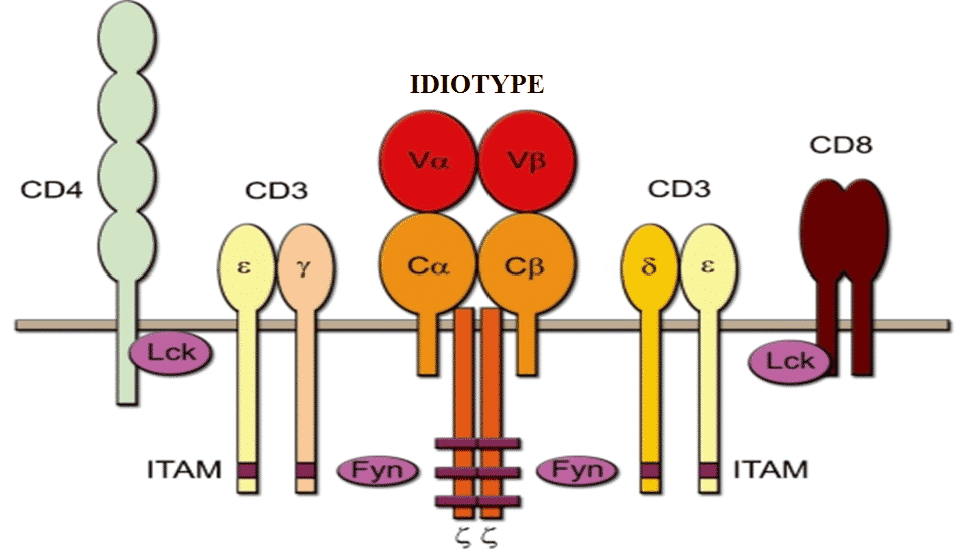
Figure 1. Schematic representation of the T-cell receptor
The role of anti-idiotypic T cells consists in regulating idiotypic T-cell activity. The concept of T-cell vaccination (TCV) is based on multiple immunizations with pathogenic T-cells to delete or inactivate targeted T-cells. TCV-induced anti-idiotypic T-cells have been shown to consist of both CD4+ and CD8+ T-cells, with fine specificity for hyper-variable TCR regions, including both CDR2 and CDR3 determinants (Zang et al. 2000). The CD8+ T-cells may be cytotoxic and/or inhibitory for CD4+ target T-cells (Buenafe et al. 2004). By secreting a variety of cytokines, including IL-4 and IL-10, the CD4+ T-cells may down-regulate the function of pathogenic immune cells. Moreover, the TCV-stimulated, CD4+FoxP3+T-regulatory (Treg) cells can inhibit the activation of target T-cells through cognate T-T interactions (Seledtsov et al. 2010; Vandenbark & Abulafia-Lapid 2008).
T-cell Immunotherapy (T-cell vaccination) can be useful for the treatment of the following diseases:
- Autoimmune diseases:
- Multiple sclerosis
- Rheumatoid arthritis
- Spondylitis deformans
- Psoriasis
- Autoimmune vasculitis.
- Allergic polyantigenic diseases:
- Allergic rhinitis
- Allergic conjunctivitis
- Urticaria
- atopic dermatitis.
Procedure (see Figure 2):
- Venous blood (300-400 ml) is taken once from a patient during exacerbation of disease.
- Autologous individual polyclonal T-cell vaccine is prepared using our state-of-the-art 5 day-long procedure, which involves isolation of pathogenic T-lymphocytes from the blood and their subsequent activation in order to enhance their immunogenicity.
- The induction course of immunotherapy consists of 6-8 subcutaneous immunizations, which are accomplished within 2 months.
- The treatment is carried out in outpatient settings.
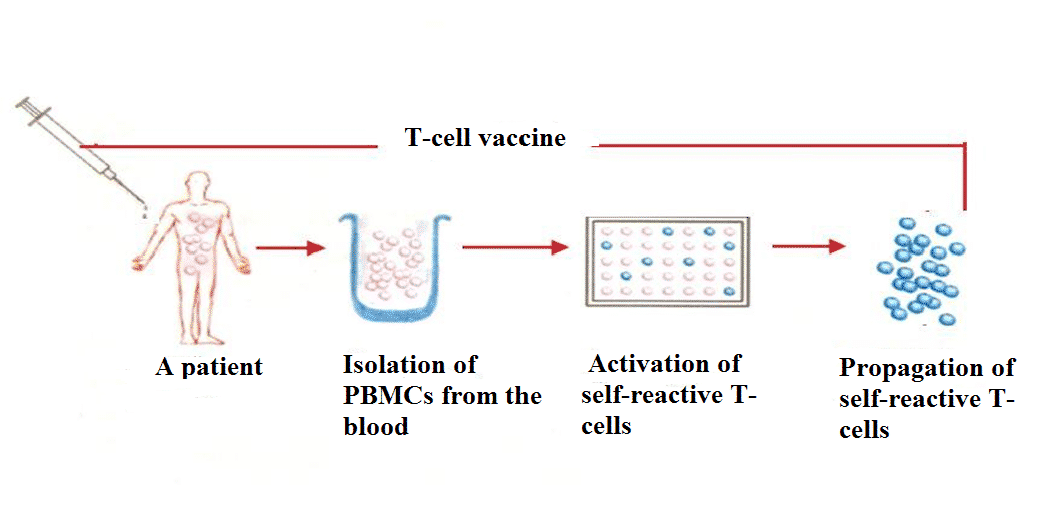
Figure 2. Preparation of polyclonal T-cell vaccine.
The above-described technology allows to keep the original proportions of pathogenic T-cells. This means that the particular T-cells that play the most important role in the development of the disease will be making the most significant contribution to induction of anti-idiotypic immune responses to the patient-specific TCV formulation. According to our observations, a disease remission can develop within first few weeks after treatment onset.
Main benefits and advantages of Т-cell autoimmunotherapy:
- Selectivity in inactivating pathogenic lymphocytes.
- The absence of both significant side effects and absolute contraindications.
- Induction of durable remission caused by the generation of anti-idiotype immune memory.
- The possibility of re-treatment if and when new pathogenic T cells appear, as judged from disease exacerbation.
- A broad possibility for combining Т-cell autoimmunotherapy with other treatments, including physiotherapy.
See our publications for more information.
T-cell Immunotherapy (T-cell Vaccination) for Multiple Sclerosis
Multiple sclerosis is a demyelinating autoimmune disease targeting brain tissues. Self-reactive T lymphocytes targeting brain-associated antigens play a major role in the pathogenesis of multiple sclerosis. Standard treatment of multiple sclerosis patients is based on nonspecific (nonselective) immunosuppressants/immunomodulators, such as hormones, interferons and others, which are, indeed, incapable of interrupting immunopatological events underlying the disease and are frequently associated with serious side effects.
Autologous T-cell vaccination exploits natural immunoregulatory mechanisms of patients aiming to achieve a selective inactivation of pathogenic autoreactive Т lymphocytes. A total of 32 patients with remittent multiple sclerosis were enrolled in a clinical trial. As shown in Figures 1 and 2, T-cell vaccination dramatically reduced the exacerbation rate and significantly improved the neurological status in patients during the first 2 years after treatment (P <0.05).
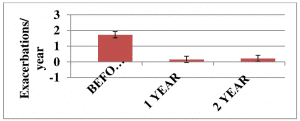
Figure 1. The exacerbation rate in patients (n=32) with remittent multiple sclerosis before and after immunotherapy onset.
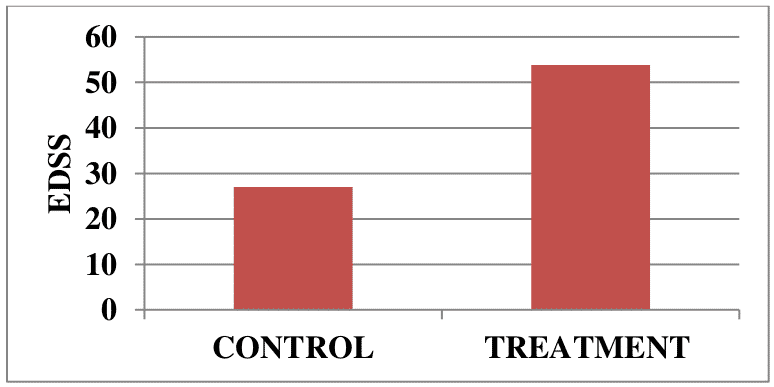
Figure 2. Neurological status in patients (n=32) with remittent multiple sclerosis before and at 2 years after immunotherapy onset.
Disease stabilization and/or neurological improvements were documented in 29/32 (~90%) patients from the treatment group, while disease progression was observed only in 3/32 (~10%) patients.
A total of 39 patients with progressive secondary (chronic) multiple sclerosis were enrolled in another clinical trial. Disease progression was stopped in 21/39 vaccine-treated patients (53.8%), compared with 6/22 (27%) control patients with no signs of disease progression, during a 2 year follow-up. Neurological improvements were detected in 5 vaccinated patients and none in the control group.
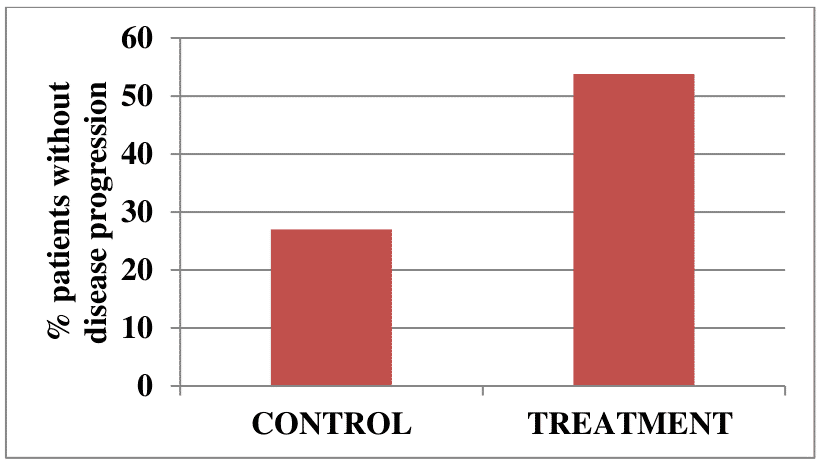
Figure 3. Percent of patients with chronic multiple sclerosis with no signs of disease progression over 2 years in the treatment and control groups.
Overall, our clinical data suggests that the nervous system of multiple sclerosis patients retains certain regenerative potential that can be activated when disease progression is stopped. We also conclude that autologous T-cell vaccination could be clinically effective in early and advanced stages of the disease.
T-cell immunotherapy (T-cell vaccination) for rheumatoid arthritis
Pro-inflammatory autoreactive T lymphocytes specific for cartilage-associated antigens play a pivotal role in the development of rheumatoid arthritis. Standard treatment of rheumatoid arthritis is based on nonsteroidal anti-inflammatory drugs (NSAIDs), nonbiologic and biologic disease-modifying antirheumatic drugs (DMARDs) and non-specific (non-selective) immunosuppressants (hormones, cytostatic agents and others). This treatment fails to interrupt the underlying immunopathological events and is often associated with serious complications.
T-cell autovaccination-based technology is aimed to stimulate patient’s immune responses, which selectively inactivate pathogenic self-reactive Т lymphocytes.
Forty-two patients (age 28–58 y; 37 women, 5 men) with rheumatoid arthritis (disease history ≥2 years) were subjected to the T-cell vaccination treatment. Our clinical data (Table 1) showed that T cell vaccination of rheumatoid arthritis patients (n = 42) resulted in apparent clinical improvements over a 2 year follow-up .
Table 1. Clinical parameters of rheumatoid arthritis patients.
| Parameter | Follow-up time (months) | |||||
| 0 (n=42) | 3 (n=42) | 6 (n=42) | 12 (n=42) | 18 (n=32) | 24 (n=22) | |
| COE (mm/h) | 38.41±2.20 | 31.12±1.94** | 22.81±1.26** | 21.75±1.60** | 20.19±1.74** | 21.14±2.12** |
| Hb (g/L) | 107.80±1.45 | 113.70±1.25** | 120.81±1.01** | 125.20±0.98** | 126.42±1.17** | 126.46±1.88** |
| DAS 28 | 5.92±0.13 | 4.93±0.16** | 3.34±0.18** | 2.99±0.17** | 2.96±0.21** | 3.12±0.27** |
In conclusion. rheumatoid arthritis patients with early and advanced stages of the disease can obtain significant clinical benefits due to the application of autologous T cell vaccination.
Related scientific publications
- Vaccination against T-cell epitopes of native ApoB100 reduces vascular inflammation and disease in a humanized mouse model of atherosclerosis.
Gisterå A, Hermansson A, Strodthoff D, Klement ML, Hedin U, Fredrikson GN, Nilsson J, Hansson GK, Ketelhuth DF. J Intern Med. 2017 Apr;281(4):383-397. doi: 10.1111/joim.12589. Epub 2017 Feb 13.
https://www.ncbi.nlm.nih.gov/pubmed/28194913 - The Impact of T Cell Vaccination in Alleviating and Regulating Systemic Lupus Erythematosus Manifestation.
Huang L, Yang Y, Kuang Y, Wei D, Li W, Yin Q, Pang J, Zhang Z. J Immunol Res. 2016;2016:5183686. doi: 10.1155/2016/5183686. Epub 2016 Dec 4.
https://www.ncbi.nlm.nih.gov/pubmed/28044142 - Autoantigen-specific immunosuppression with tolerogenic peripheral blood cells prevents relapses in a mouse model of relapsing-remitting multiple sclerosis.
Kleist C, Mohr E, Gaikwad S, Dittmar L, Kuerten S, Platten M, Mier W, Schmitt M, Opelz G, Terness P. J Transl Med. 2016 May 1;14(1):99. doi: 10.1186/s12967-016-0860-6.
https://www.ncbi.nlm.nih.gov/pubmed/27131971 - The role of autoreactive T cell in the pathogenesis of rheumatoid arthritis and implications for T cell targeted vaccine therapy.
Wang D, Li Y, Liu Y, Shi G. Minerva Med. 2015 Jun;106(3):157-67. Review.
https://www.ncbi.nlm.nih.gov/pubmed/26057192 - T cell vaccination in systemic lupus erythematosus with autologous activated T cells.
Li ZG, Mu R, Dai ZP, Gao XM. Lupus. 2005;14(11):884-9.
https://www.ncbi.nlm.nih.gov/pubmed/16335580
