Antitumor cellular vaccination includes administration of dendritic cells and macrophages pulsed with xenogeneic differentiation antigens, which could be supplemented with adoptive T-cell therapy. In this formulation context, the interaction between professional antigen-presenting cells and activated T-lymphocytes empowers this particular combined vaccine preparation with a high clinical potential to break the immune tolerance to the tumor, thus, preventing further cancer development.
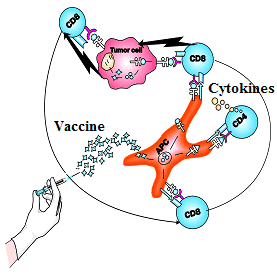
To date, we have developed a novel ‘mix and match’ immunotherapeutic technology in the laboratory, which essentially is based on different compositions of xenogeneic differentiation antigens specifically adopted for treatment of various tumor diseases in the clinic. Those compositions include both common tumor-associated and tissue-specific antigens. Small structural differences of the xenogeneic antigens from their human analogs render these antigens highly immunogenic and capable of inducing immune responses in patients with advanced stages of disease when tumor-derived immunosuppression becomes a significant player in advanced cancer patients. We envisage that upon administration into the human body, xenogeneic cell membranes are opsonized with natural antibodies and phagocytized by professional antigen-presenting cells (macrophages, dendritic cells) via an FcR-dependent mechanism. These are the mechanisms that underlie the ability of vaccine antigens to generate antitumor T-cell responses. Our well-established original immunotherapeutic technology including : (i) vaccinations with macrophages and dendritic cells pulsed with xenogeneic differentiation antigens, and (ii) generation of activated effector T cells with subsequent adoptive transfer, allows to intensify (accelerate) the development of anti-tumor immune responses in cancer patients. Importantly, the novel ‘mix and match’ immunotherapeutic technology makes it possible to apply different xenogeneic vaccine compositions for treatment of various tumor diseases in individual patients. According to our clinical observations, active cellular immunotherapy possesses a selective cytoreductive activity, and can provide a long-lasting antitumor effect in cancer patients; it is also devoid of serious complications which are often encountered when applying other cancer treatments.
Diseases:
- Melanoma
- Renal cancer
- Colorectal cancer
- Brain tumors
- Prostate cancer
- Lung cancer
- Breast cancer
- Gastric cancer
- Thyroid carcinoma
- Ovarian carcinoma
- Pancreas cancer
Procedure:
- Venous blood (300-350 ml) is taken once from a patient or his closely related donor.
- Preparation of antitumor immune cells using our established 7 day protocol.
- Vaccine cells are prepared (5-7 doses), cryopreserved and stored in liquid nitrogen (-1960 С) until use.
- After thawing, cell preparations are administered intramuscularly or intravenously, as required.
- The treatment is carried out in outpatient or inpatient settings.
Main benefits and advantages of cellular immunotherapy:
- High immunogenicity of xenogeneic antigens allowing to generate antitumor immune responses both at early, and advanced stages of the disease.
- The availability of various sets of antigens (there are currently 10 common sets) with proven clinical efficacy in breaking the immune tolerance to the particular type of tumor.
- The ability to generate a long-lasting antitumor effect.
- Absence of both serious side effects and absolute contraindications.
- The technology does not require autologous (received from the patient) tumor material.
- Low invasiveness of treatment.
- Single blood sampling and the possibility of optimizing the treatment regimen for each individual patient.
- The possibility of a complete recovery.
Related scientific publications:
- Pilot study of safety and feasibility of DNA microseeding for treatment of spontaneous canine melanoma.
Zuleger CL, Kang C, Ranheim EA, Kurzman ID, Macklin MD, Newton MA, Wolchok JD, Vail DM, Eriksson E, Albertini MR. Vet Med Sci. 2017 May 22;3(3):134-145. doi: 10.1002/vms3.65. eCollection 2017 Aug.
https://www.ncbi.nlm.nih.gov/pubmed/29067210 - Evaluation of a xenogeneic vascular endothelial growth factor-2 vaccine in two preclinical metastatic tumor models in mice.
Denies S, Leyman B, Huysmans H, Combes F, Mc Cafferty S, Cicchelero L, Steppe M, De Temmerman J, Sanders NN. Cancer Immunol Immunother. 2017 Dec;66(12):1545-1555. doi: 10.1007/s00262-017-2046-3. Epub 2017 Aug 3.
https://www.ncbi.nlm.nih.gov/pubmed/28776079 - Anticancer effect and immunologic response to xenogeneic embryonic proteins in mice bearing Ehrlich solid carcinoma.
Symchych TV, Fedosova NI, Karaman ОМ, Yevstratieva LM, Potebnia HP. Exp Oncol. 2017 Mar;39(1):42-48.
https://www.ncbi.nlm.nih.gov/pubmed/28361853 - Immunogenicity and safety of xenogeneic vascular endothelial growth factor receptor-2 DNA vaccination in mice and dogs.
Denies S, Cicchelero L, Polis I, Sanders NN. Oncotarget. 2016 Mar 8;7(10):10905-16. doi: 10.18632/oncotarget.7265.
https://www.ncbi.nlm.nih.gov/pubmed/26871296 - Safety of administering the canine melanoma DNA vaccine (Oncept) to cats with malignant melanoma – a retrospective study.
Sarbu L, Kitchell BE, Bergman PJ. J Feline Med Surg. 2017 Feb;19(2):224-230. doi: 10.1177/1098612X15623319. Epub 2016 Jul 10.
https://www.ncbi.nlm.nih.gov/pubmed/26685147
